Learn more about our latest releases
May 2, 2024, Release Notes
Customized content in the homepage; Enhanced Comparative Table Builder; New Exports; OFF-X Knowledge Base; Updated RWE functionality; Updated FDA SBAs navigation; Enhanced MHRA drug approval content.
February 21, 2024, Release Notes
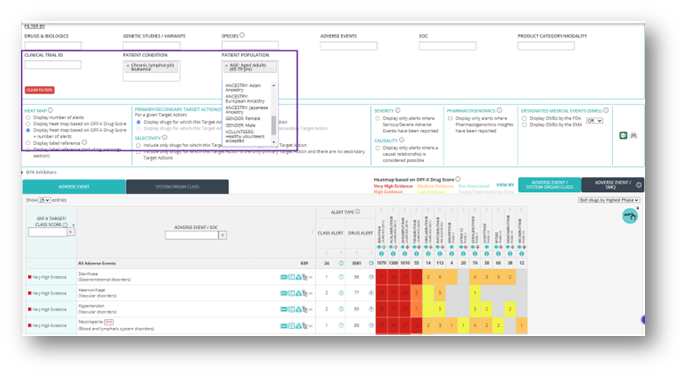
Enhanced export functionalities; Set up customized notifications; Comparative drug safety views with frequency values; Contextual FAQs; Classification of CAR-T cell therapies based on viral vector.
November 15 2023 Release Notes
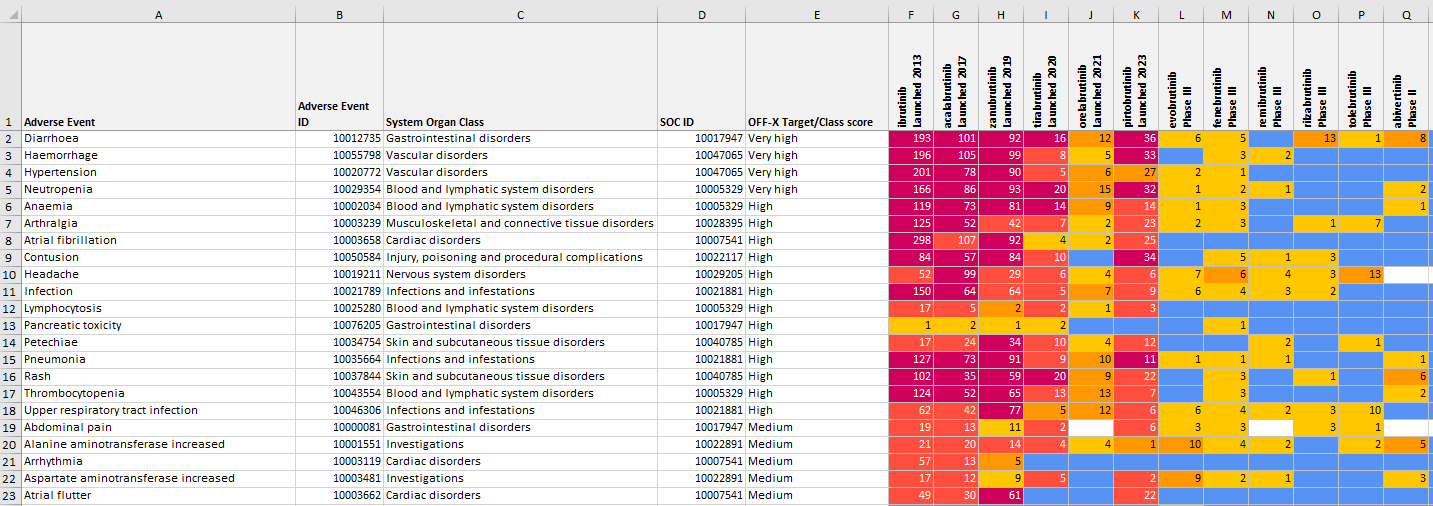
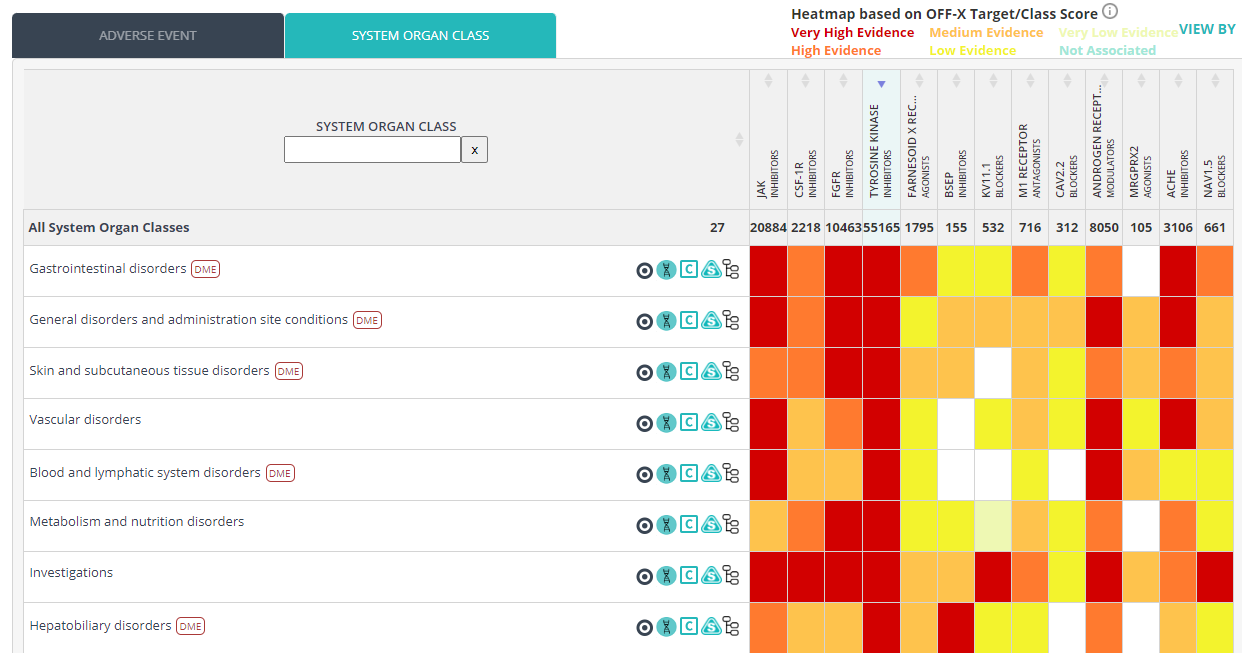
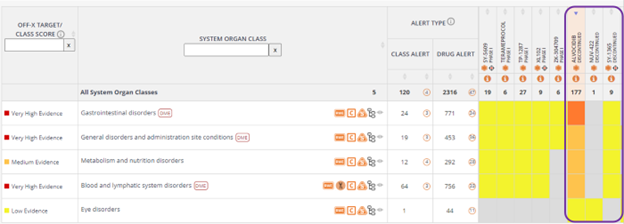
Update and improvement of the OFF-X Target/Class algorithm; Comprehensive curation of the full archive of FDA labels
June 14 2023 Release Notes
New clinical trial metadata and filters; seamless navigation to Cortellis Trials Intelligence; improvement of the OFF-X Drug Score; all FDA labels since 1965.
May 10 2023 Release Notes
Enhanced coverage of discontinued drugs; New content added to Secondary Pharmacology Panels; Updated Safety Maps; 200 FDA labels approved between 1980-1999; Updated links to ChEMBL from drug records.
February 22 2023 Release Notes
Viral vector-centric safety assessment of gene therapies; Updated and enhanced PubMed text mining data to complement curated safety alerts; Completion of the manual curation of the archive of PMDA drug approval packages.
OFF-X December 21 2022 Release Notes
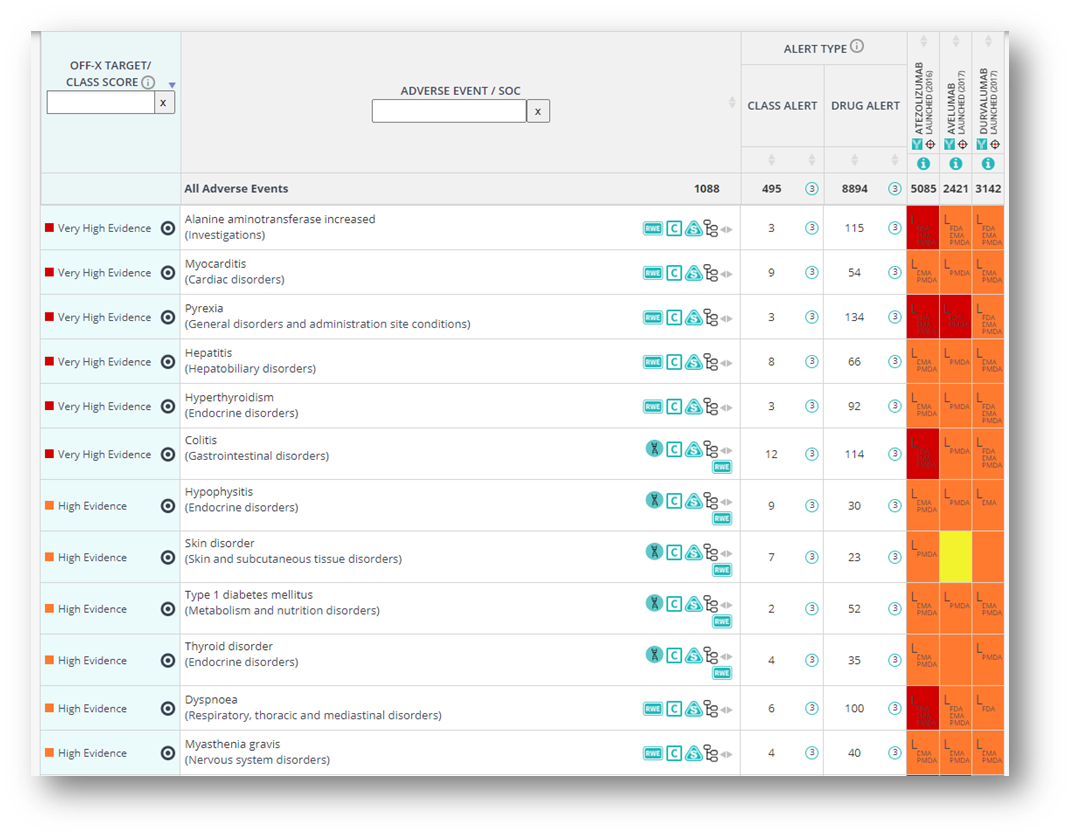
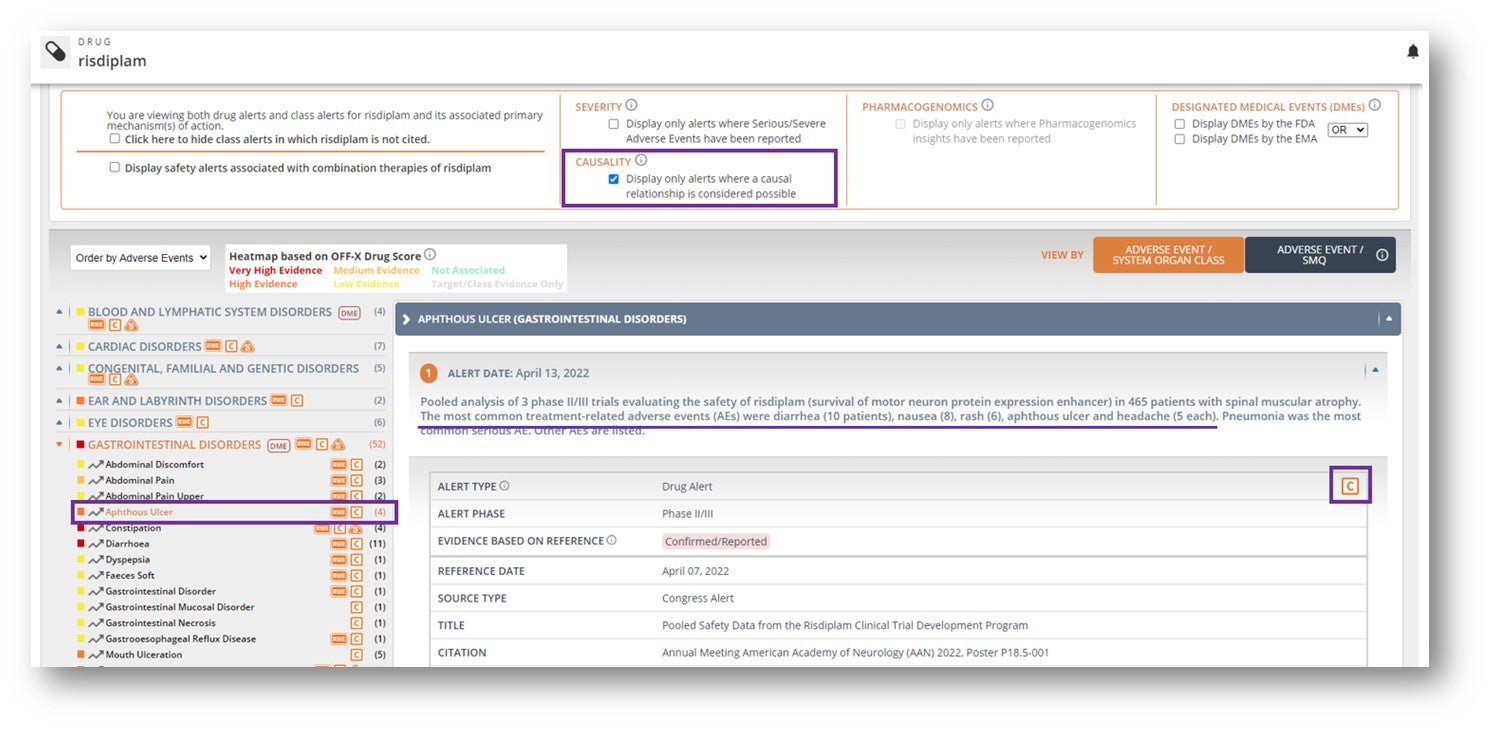
Causality metadata & filter to identify treatment-related adverse events; Frequencies of adverse events; Safety data of vaccines approved by the FDA and EMA; Enhancement of the Secondary Pharmacology Panels tool.
OFF-X November 16 2022 Release Notes
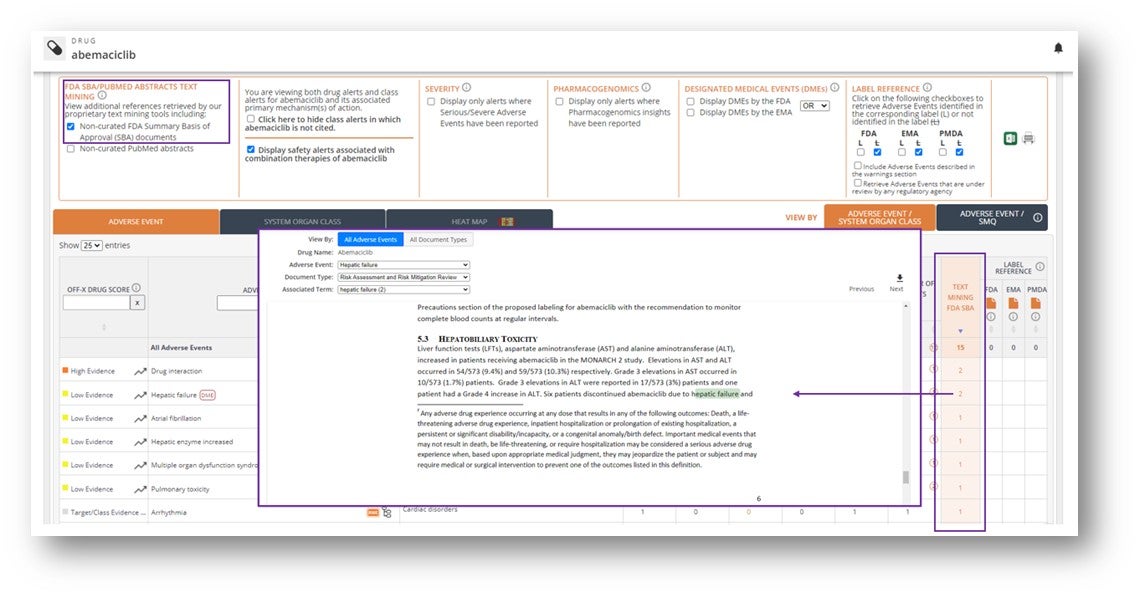
New analytics page grouping the analytic tools by use case; New header, sub-header and search bar; Enhancement of the Drug Label Analyzer tool; Text mining results from FDA Summary Basis of Approval documents shown by default in all views.
OFF-X October 5 2022 Release Notes
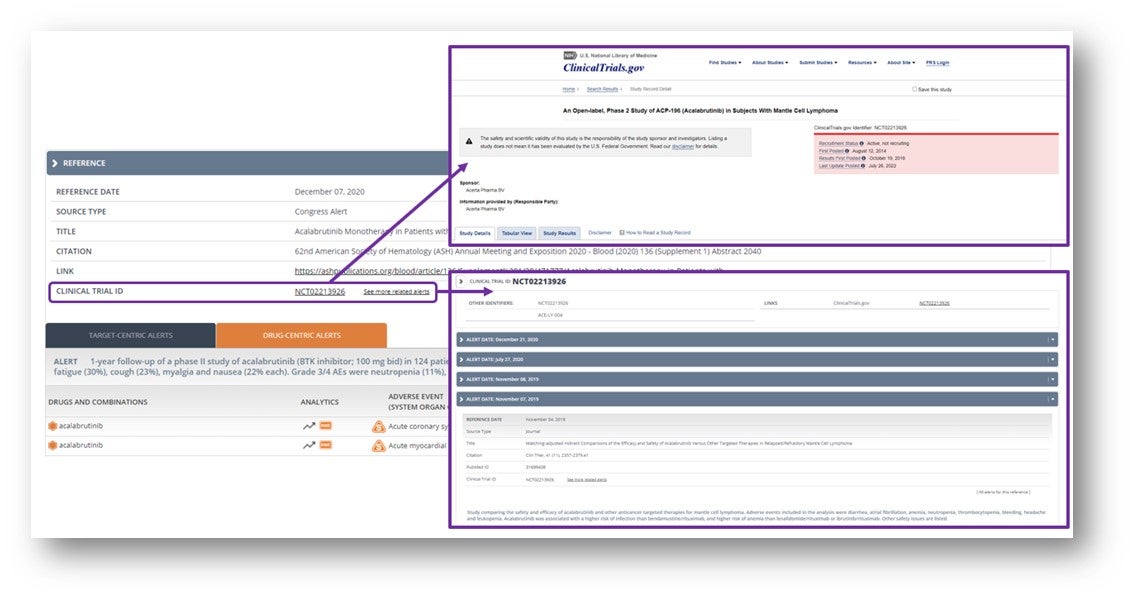
Links to clinicaltrials.gov; Clinical trial record to access different publications covering the same clinical study; Improvement of the OFF-X Drug Score; Webinars and recorded training sessions
OFF-X September 7 2022 Release Notes
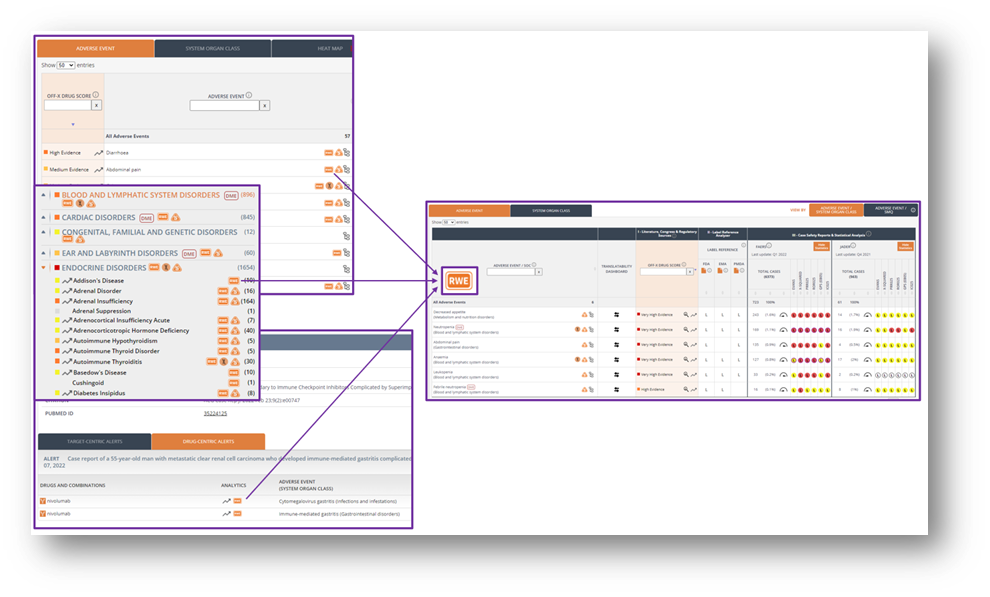
Further integration of the RWE Analysis Dashboard in pharmacovigilance use cases; Shortcut to FDA Summary Basis of Approval documents; Expanded drug mapping between OFF-X and Cortellis Competitive Intelligence
OFF-X July 27 2022 Release Notes
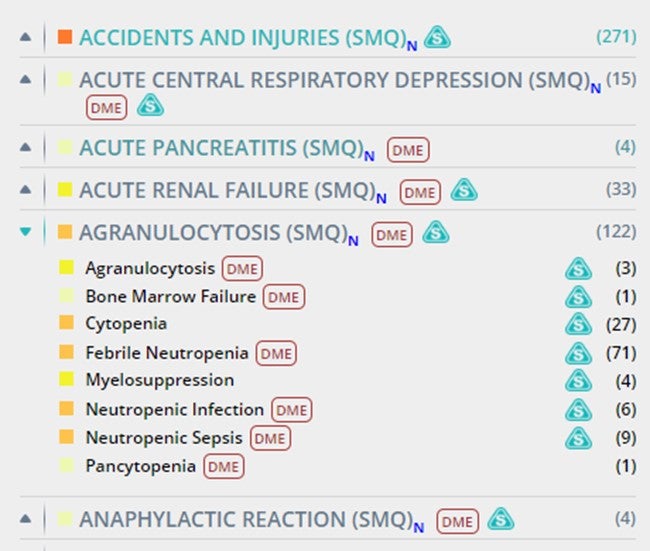
Enhancement of the Safety Alerts section for the assessment of PV signals, including SMQ navigation and links to analytics; Enhanced SMQ navigation in the RWE Analysis Dashboard; Expansion of the retrospective coverage of FDA SBA documents.
OFF-X June 29 2022 Release Notes
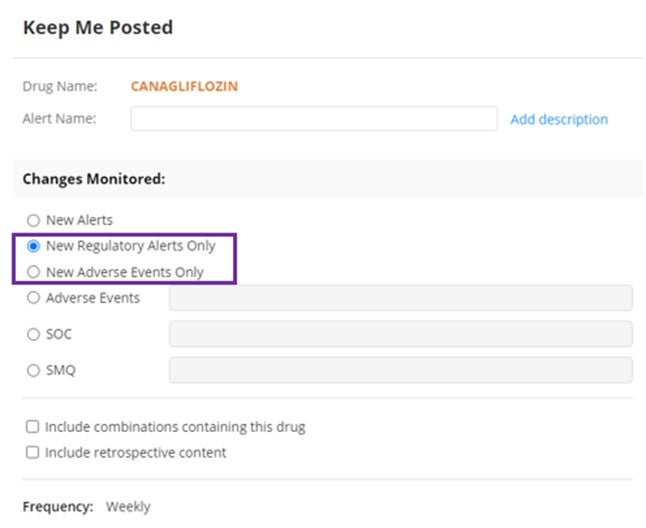
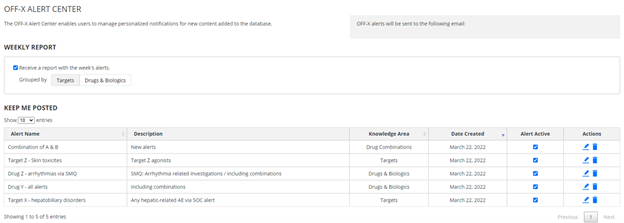
Label changes and new adverse events notifications added to Keep Me Posted alerts; OFF-X Drug and Target/Class Score added to the Safety Alerts section; Enhanced filtering functionalities; Retrospective analysis of PMDA approval packages.
OFF-X May 24 2022 Release Notes
Further integration of safety data extracted from FDA Summary Basis of Approvals (SBA); Enhanced navigation of the FDA SBA text mining results; New filter in the RWE dashboard to exclude adverse events reported in FDA SBAs; Short-cut to the safety alerts of drug’s secondary targets; Seamless navigation between OFF-X and other Clarivate products.
OFF-X April 5 2022 Release Notes
Customization of the alerts sent to the user’s mailbox; New analytics to allow the comparison of the safety profile of drugs and their targets; Integration of safety data extracted from FDA Summary Basis of Approvals via text mining methods.
OFF-X February 2 2022 Release Notes
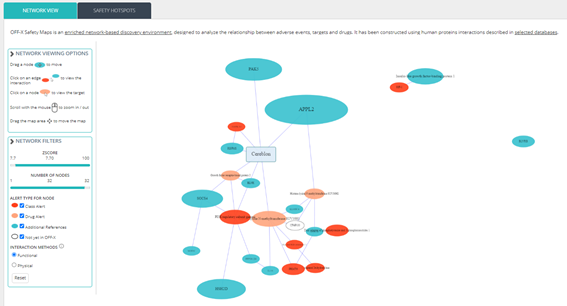
New Comparative Target Safety Evidence tool to easily assess the safety liabilities associated with the pharmacological modulation of different targets
OFF-X December 16 2021 Release Notes
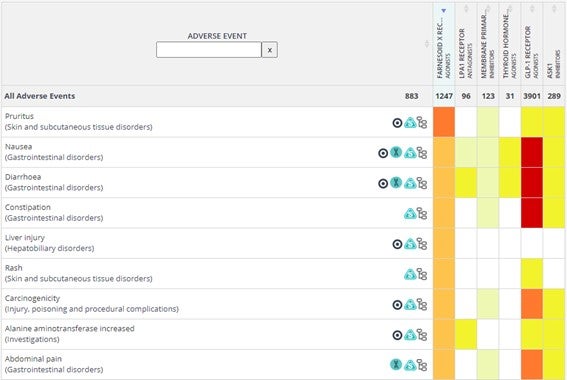
Usability enhancements with new filter options to quickly identify the adverse events of interest; Comparative Drug Safety Evidence and Master view heatmaps enhancements; FAERS and JADER content update.
November 9 2021 Release Notes
Comparative Drug Safety Evidence view enhancements and content updates (Safety Maps and Real World Evidence dashboards)
September 21 2021 Release Notes
New Real World Evidence analysis dashboard including reports from FAERS and JADER databases to validate and analyze potential pharmacovigilance signals.